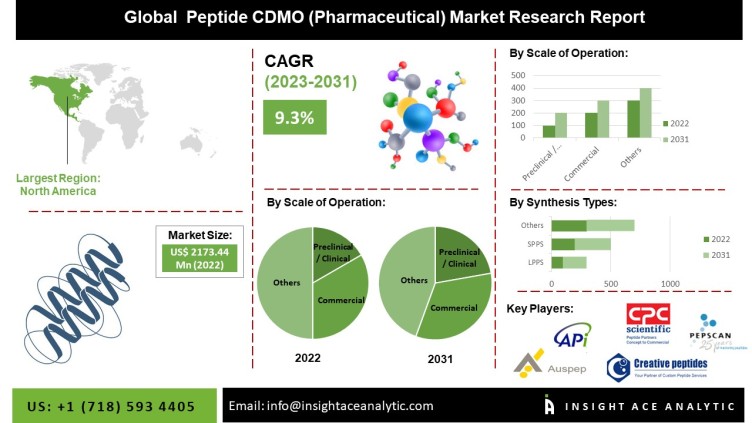
According to the latest market research report, the global Peptide CDMO (Pharmaceutical) Market Size is valued at 2173.44 Million in 2022 and is predicted to reach 4784.50 Million by the year 2031 at a 9.3 % CAGR during the forecast period for 2023-2031.
Get Free Sample Copy of Report : https://www.insightaceanalytic.com/request-sample/1202
Peptides are small protein molecules that contain fewer amino acids compared to proteins. Peptide therapeutics play an essential role in addressing unmet medical needs. Several peptide drugs have reached the market for treating different diseases, including cancer, diabetes, osteoporosis, chronic pain, multiple sclerosis, and HIV infection. Peptide CDMO is considered an emerging market due to its excellent peptide therapeutic properties and promising potential for the contract API manufacturing industry.
Factors such as the developing healthcare infrastructure, increasing medical applications of peptides, rising healthcare expenditures, emerging peptide design technologies, growing usage of peptides for drug development, rising R&D investments for peptide-based therapies, high prevalence of chronic diseases, and the use of bioinformatics and systematic biological approaches for peptide designing are predicted to drive the peptide CDMO (pharmaceutical) market during the forecast years.
The high prevalence of chronic and infectious diseases leads to increased drug demands. Hence, pharmaceutical companies began to opt for peptides and CDMOs services to manage the product manufacturing burden. Peptides are used due to their high specificity, tolerability, and ease of manufacturing, and contract manufacturing services save time and enhance production efficiency.
Likely to surge the business growth opportunities for the peptide CDMO (pharmaceutical) market over the projected period.
However, factors such as the expensive CDMOs services and the need for specialized expertise for peptide production are estimated to impede the market development over the estimated timeframe.
Request for ToC/Proposal: https://www.insightaceanalytic.com/report/global-peptide-cdmo-pharmaceutical-market-/1202
Regionally, North America, followed by Europe, is projected to lead the peptide CDMO (pharmaceutical) market during the forecast period (2022-2030), owing to the well-developing CDMOs, availability of peptide synthesis technologies, and the surging demand for peptides and drugs to treat chronic conditions.
Key market players operating in the peptide CDMO (pharmaceutical) market include AmbioPharm – A Global Peptide CDMO, Auspep, Bachem, BCN Peptides, CPC Scientific Inc., CBL- Chemical and Biopharmaceutical Laboratories, ScinoPharm Taiwan Ltd, Olon, Belyntic GmbH, Ferring Pharmaceuticals , NUMAFERM, Hybio Pharmaceutical, Provepharm life solutions, Enzene Biosciences Ltd , Ardena Holding, Stelis Biopharma, Piramal Pharma Solutions, Space Peptides Pharmaceutical, Creative Peptides, CSBio, Corden Pharma – A Full-Service CDMO, PolyPeptide Group, Hybio Pharmaceutical, Peptide Institute, Almac Group, chengdu shengnuo Peptide Company, CreoSalus Inc, Vivitide, Senn Chemicals AG,, among others.
Key developments in the market:
In January 2022, CordenPharma collaborated with PeptiSystems, a Swedish-based developer of peptide and oligonucleotide therapeutic process development and manufacturing instruments, to reduce the footprint impact and improve the Process Mass Intensity (PMI) of peptide manufacturing processes.
In December 2021, Pepscan extended its column purification technology with a mid-sized column packer. This technology significantly reduces purification time and provides optimal flexibility when synthesizing peptides.
In September 2021, CEM Corporation and AmbioPharm, Inc. partnered to produce GMP peptides for the global market. The partnership will involve an exclusive1 use of CEM’s large-scale microwave peptide synthesis technology to produce GMP peptides up to multi-kilogram quantities using proprietary scalable reactors. Also, it will enable rapid and efficient production of peptide new chemical entities (NCE’s) using the latest technologies available.
In July 2021, CordenPharma expanded its peptide manufacturing capacity in CordenPharma Colorado, their GMP API facility in Boulder, CO (US). CordenPharma Colorado is the largest worldwide peptide API producer leading the global peptide market.
Obtain Full Report @ https://www.insightaceanalytic.com/enquiry-before-buying/1202
Market Segments
Global Peptide CDMO (Pharmaceutical) Market, by Scale of Operation, 2023-2031 (Value US$ Mn)
Preclinical / Clinical
Commercial
Global Peptide CDMO (Pharmaceutical) Market, by Method Used, 2023-2031 (Value US$ Mn)
Chemical Synthesis Method
Non-Chemical Synthesis Method
Global Peptide CDMO (Pharmaceutical) Market, by By Applications, 2023-2031
Other Polypeptide APIs Products
peptide synthesis companies
| Polypeptide APIs Products |
| US-DMF LIST |
| Beauty peptides |
| Chinese cGMP APIs |
| Mexico Registered APIs |
| Research Peptide APIs for Regulatory Market |
| Polypeptide Preparation |
| Kaijie bio medicine Peptide APIs |